Developing an Effective Lipid Nanoparticle Formulation Process for Small Molecule and Biologicals Delivery
Lipid nanoparticles (LNPs) are a versatile formulation technology used by drug development teams to deliver a variety of small/large molecules, peptides, and biologicals. Due to a lipid’s unique properties and excellent biocompatibility, LNPs are used in pharmaceuticals, nanomedicines, vaccines, nutritional supplements, and diagnostics by different routes of administration, such as oral, topical, pulmonary, and parenteral injection.
LNPs have emerged as a drug delivery system for biologicals. This is particularly true for COVID-19 mRNA vaccines, as LNPs serve a vital role in transporting mRNA into the target cells.
For these reasons, CDMOs with LNP expertise can prove to be a valuable partner who can help push drugs through the pipeline more efficiently. Ascendia Pharmaceuticals team of scientists has established a general guide and practice process to select LNPs for different types of therapeutic moiety (figure 1).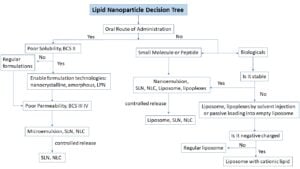
Liquid-Form LNP Types
Depending on the surfactant level, LNPs can be classified as a microemulsion or nano-emulsion.
Microemulsions – For oral administration, LNPs can be frequently presented as a lipid concentrate, referred to as a microemulsion. In this delivery method, the lipid concentrate is diluted by GI fluid and spontaneously form lipid nanoparticles. Due to lipophilicity of the lipidic material, oral LNPs have traditionally been used to enhance solubility and bioavailability of small molecule compounds and peptides that have solubility and cell permeability issues.
LNP-based drug delivery systems enhance the oral bioavailability of drugs via three main mechanisms:
♦ increasing dissolution and solubility by pre-dissolving drugs in lipid carriers
♦ increasing drug permeability in the GI tract by inhibition of P-gp and other efflux transporters
♦ bypassing the first-pass metabolism of the drug through the lymphatic absorption processes
Lipid-based drug delivery systems have the potential to decrease the food-effect. Increased reproducibility of the pharmacokinetic profile of orally administered drugs is also achieved by reducing erratic absorption.
Lipid concentrate can be characterized into five categories. For insoluble drugs, Type II/IIIA: Self-Emulsifying Drug Delivery Systems (SEDDS) and Type IIIB: Self-Microemulsifying Drug Delivery Systems (SMEDDS) are the most common. Both form microemulsions that are thermodynamically stable.
Lipid concentrate for oral formulations can be processed by mixing, dissolving, and filling into bottles or liquid-filled capsules. Hard gelatin/HPMC capsules are frequently chosen for lipid-based oral formulations, from early to commercial stages of development and different options for trade dress.
Nano-emulsions – Nano-sized droplets dispersed in a continuous phase form nano-emulsions. Oil-in-water (O/W) nano-emulsions have gained significant application in the drug delivery of lipophilic drugs or nutritional supplements by parenteral, oral, and topical routes of administration. An alternative, water-in-oil (W/O), is increasing in application for delivery of hydrophilic small molecules and biologicals.
Nano-sized emulsions can provide unique solutions to address drug solubility, permeability, and stability. Nano-emulsions contain considerably less surfactants, which makes them meta-stable and more susceptible to Ostwald ripening. Nano-emulsions also require greater kinetic formation energy, and are usually prepared using high-pressure homogenization, microfluidics, or ultrasonic generators.
A well-formulated nano-emulsion will maintain its physical-chemical stability through its shelf-life (2-5 years). They are also preferred over microemulsions for human use, in part because nano-emulsions reduce injection pain and thrombophlebitis.
Comparting Solid and Liquid Lipid Nanoparticles
 Solid lipid nanoparticles (SLNs) are an alternative system to emulsion dosage forms. Some SLNs have excellent biocompatibility and high bioavailability. Their nano-size and large surface area are similar to emulsions. SLNs can also maintain high drug loading and sustained delivery of drug from its matrix.
Solid lipid nanoparticles (SLNs) are an alternative system to emulsion dosage forms. Some SLNs have excellent biocompatibility and high bioavailability. Their nano-size and large surface area are similar to emulsions. SLNs can also maintain high drug loading and sustained delivery of drug from its matrix.
Structure lipid nanoparticles (NLCs) were developed to overcome the potential issues with SLNs. NLCs can increase drug loading by combining solid lipids with small amounts of liquid lipids or by the formation of an amorphous type NLC by introduction of special lipids, such as hydroxyl stearate and isopropyl myristate, to the solid lipid.
Like traditional emulsion formulation, SLNs/NLCs are made up of solid and/or liquid lipids, emulsifier, and water. The lipids used may be triglycerides, partial glycerides, fatty acids, cholesterol, and waxes.
Process for SLN/NLC
Similar to the emulsion process, high-shear, high-pressure homogenization can be utilized for SLNs/NLCs. Normally, homogenization is performed at temperatures above the melting point of the lipid to form a smaller and more uniform droplet size distribution. In some cases, cold homogenization is performed to maintain the solid state of lipid and to avoid segregation of drug and lipid due to drug migration to the lipid surface during the homogenization process.
Liposomes and Lipoplexes
Liposomes consist of lipid bilayers that range from 20 nm to ~1000 nm. Their unique structure enables hydrophilic drugs to be loaded into its aqueous interior and hydrophobic drugs in its lipid bilayer. Liposomes can also be classified into two categories: multilamellar vesicles (MLVs) or unilamellar vesicles. The latter can be further divided into large unilamellar vesicles (LUVs) and small unilamellar vesicles (SUVs).
Liposomes for small molecule delivery are most commonly derived from phospholipids. These include phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylserines (PS), phosphatidylglycerols (PG), and helper lipids, such as cholesterol and PEG-Lipid.
Phospholipids exist in a liquid crystalline phase or gel state, depending on the phase transition temperature during manufacturing. In addition, the effect of formulation on liposome stability, storage temperature, and drug release is related to the phase transition temperature. Drug release from the liposome is accelerated at a temperature above the phase transition temperature.
Typically, RNA liposomes consist of cationic lipids and DSPC as the main ingredients, as well as helper lipids, such as cholesterol and PEG-lipid. Helper lipids aid in physical stability, enhance blood circulation time, and improve RNA delivery. Lipoplexes that formed by mixing preformed cationic lipid liposomes with anionic siRNA in an aqueous environment can be used in certain projects.
Cationic Lipids
Cationic lipids or ionizable cationic lipids become critical ingredients to improve RNA encapsulation efficiency, for RNA liposomal delivery. Additionally, cationic lipids can reduce RNA degradation in neutral body fluid because of complexation between RNA and cationic lipids. They also promote endosomal escape inside the cell.
A newer class of organic compounds is ionizable cationic lipids. Their ability to change their charge as a function of pH is how they are characterized. At a low pH, they are protonated and carry a positive charge. At neutral pH, they are neutral without charge. The positive charge at a low pH helps encapsule RNA with good efficiency. The neutral charge of the LPN in the physiology pH helps lower RNA exposure to the body fluid that minimizes RNA degradation.
When LPN is at the target cell, a low pH environment helps enhance LPN cellular uptake. This is due to interaction of cationic lipid with negative change cell membrane that breaks down the bio-layer membrane and helps RNA in endosomal escape and release into the target cell organ.
Liposome Preparation Methods
Drug development teams can make liposomes in several ways. One popular method is film hydration. It involves drying lipids from an organic solvent, rehydrating the lipids in aqueous media, formation of liposome, loading the liposomes by active or passive method (if necessary), purification of liposomes by ultrafiltration method, sterile filtration, lyophilization (when required), and fill and finish into a vial.
Another common approach is solvent injection. The process begins by mixing the lipid ethanol solution with an aqueous solution at a certain ratio/speed followed by the similar steps of the film hydration method.
The key for liposome preparation is the formation of liposomes after initial mixing. This step may involve high pressure, high shear mixing (homogenizer and microfluidizer), extrusion, microfluidic chip mixing, sonication, and freeze thaw. This step will determine the particle size distribution of liposomes, drug loading, liposome structure, and drug distribution in the liposome.
As you can see, the LNP formulation process is a complex process. Pharmaceutical companies have many hurdles to overcome and a single misstep can lead to expensive lost time and development. To help smoothen this journey, Ascendia Pharmaceuticals created “Drug Formulation Development Process: Notes from a CDMO” to provide expert insight into how to navigate the process.
Contact us to discuss how our team of specialists can help with your drug development pipeline.

